Customized Automatic Printing Machine,Custom Machine Automation,Custom Assembly Machines ShenZhen Jakeconn Precision Technology Co., Ltd. , https://www.jakeconn.com
# Passivation of Stainless Steel Components
Passivation of stainless steel parts is an essential process in numerous industries, including aerospace, defense, electronics, and medicine. This procedure creates a passive layer on the surface of stainless steel to boost its corrosion resistance and extend its service life. Understanding the science behind passivation, exploring the different types and techniques, and evaluating its applications can deepen our appreciation for its critical role in protecting stainless steel parts. In this article, we will explore key terms, chemical processes, and benefits of passivation, along with addressing its challenges and safety measures.
## Grasping Passivation
Passivation is a process designed to improve the corrosion resistance of stainless steel components by removing free iron particles and forming a passive layer on their surface. Free iron particles can interact with environmental factors, causing corrosion, oxidation, and discoloration of the metal. By eliminating these free iron particles and establishing a protective oxide layer, often called a passive layer, passivation ensures a cleaner surface, free from contamination, and promotes the formation of a stable oxide film.
## Key Terminology in Passivation
Free iron, if not addressed, can serve as a catalyst for corrosion. The primary goal is to remove it from the surface of stainless steel components. By doing so, passivation creates a clean surface, free from contamination, and encourages the formation of a passive film.
The passive film, also known as a protective oxide layer, is a critical element of passivation. It is a thin layer of metal oxide that forms on the surface of stainless steel components during the passivation process.
Surface contamination, such as dirt, oil, grease, and other foreign materials, can interfere with the passivation process. Before passivation, thorough cleaning and preparation of the stainless steel surface are essential to remove surface contaminants and ensure the effectiveness of the passivation treatment.
Conversion coating is another term related to passivation. It refers to the chemical treatment of stainless steel components to enhance their corrosion resistance and improve their surface finish. Passivation is a specific type of conversion coating, focused on stainless steel components to promote the formation of a protective oxide layer.
## The Chemistry Behind Passivation
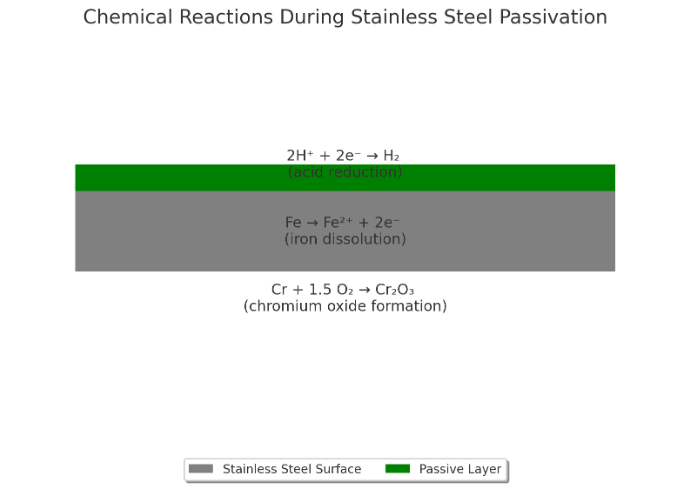
The chemistry underlying passivation involves a series of reactions that occur when stainless steel components are submerged in a passivating solution. Treatments like citric acid or nitric acid passivation initiate chemical reactions with the stainless steel surface, resulting in the formation of a passive layer. This passive layer, made up of metal oxide, acts as a protective barrier, preventing corrosion and increasing the stainless steel's resistance to environmental factors.
Understanding the chemical processes involved in passivation and how they contribute to the formation of the passive layer is crucial for optimizing the treatments.
## Types and Techniques of Passivation
Various types and techniques of passivation provide different advantages, depending on specific needs and industry applications. Two commonly used passivation methods are citric acid passivation and nitric acid passivation. Each technique has its unique characteristics and benefits, making them suitable for different scenarios.
### Citric Acid Passivation
Citric acid passivation, utilizing the natural acid found in citrus fruits, has become a favored method in industries like healthcare and food processing due to its environmental friendliness and lower toxicity compared to nitric acid passivation. This technique is not only safer to handle and dispose of but also meets strict industry standards, offering reliable corrosion resistance and a clean finish free from impurities, crucial for applications requiring high cleanliness levels. Citric acid passivation provides a viable alternative to nitric acid passivation, maintaining surface quality while aligning with specific environmental concerns and safety requirements in sensitive sectors.
### Nitric Acid Passivation
Nitric acid passivation excels in the field of metal finishing, particularly for stainless steel, because of its exceptional ability to enhance corrosion resistance, ensure surface cleanliness, and effectively remove residual acids. This process adheres to stringent industry standards, including surpassing salt spray test requirements, which assess the material's capacity to withstand corrosive environments. During nitric acid passivation, stainless steel components are immersed in a nitric acid solution, a critical step for developing a dense, protective chromium oxide layer on the metal's surface. This passive layer plays a key role in shielding the steel from various forms of corrosion, thereby significantly improving its longevity and durability.
Nitric acid passivation's stronger oxidizing properties and faster reaction time enable it to more aggressively remove iron and other contaminants from stainless steel surfaces. This leads to a quicker and more robust formation of the passive chromium oxide layer, offering enhanced corrosion resistance, particularly for high-carbon steel grades or in highly corrosive environments. Although nitric acid offers greater operational flexibility in terms of temperature and concentration, it also presents more significant safety and environmental challenges compared to the milder, eco-friendlier citric acid passivation, making nitric acid passivation a choice for applications where maximum corrosion resistance is paramount, albeit with added handling and disposal considerations.
### The Role of Oxidation in the Passivation Layer
Oxidation plays a crucial role in the creation of the passivation layer on stainless steel components. It involves the interaction of chromium, oxygen, and other elements present in the stainless steel alloy.
Chromium, an essential component of stainless steel, reacts with oxygen in the passivating solution, forming a chromium oxide layer on the surface of the stainless steel components. This process, known as oxidation, increases the corrosion resistance of the stainless steel by creating the passive layer.
The chromium oxide layer acts as a shield, preventing direct contact between the metal surface and corrosive agents. It minimizes the potential for metal degradation, such as flash attack, oxide scale formation, corrosion, and discoloration by providing a protective barrier against chemical reactions that could cause surface deterioration.
## The Passivation Process for Stainless Steel
The passivation process involves preparing and cleaning the stainless steel surface to remove contaminants. Next, applying passivation chemicals like citric acid baths or nitric acid baths promotes the formation of a chromium oxide layer for surface passivation. Finally, rinsing and drying the parts are critical for the successful completion of the passivation process to enhance corrosion resistance and durability. Regular intervals of passivation are necessary to maintain the quality of metal surfaces in manufacturing processes, ensuring the effectiveness of the passivation layer.
### Step 1: Preparation and Cleaning
Before passivating stainless steel components, key steps include thorough cleansing to eliminate contaminants. Proper preparation requires degreasing and cleaning for effective passivation. Cleaning also aids in achieving the desired surface finish. Removing surface impurities is a prerequisite before initiating the treatment. The process involves ensuring that the parts are free from any unwanted substances like iron particles or oils/grease.
### Step 2: Applying Passivation Chemicals
Enhancing stainless steel surfaces involves immersing parts in a solution to form a protective layer. The immersion time for stainless steel components in a passivation solution can vary depending on several factors, including the type of stainless steel, the passivation method used (citric acid or nitric acid), and the specific requirements of the part. It's essential to refer to ASTM standards (such as ASTM A967 for citric acid passivation and ASTM A380 for nitric acid passivation) for more detailed guidelines and to ensure that the process meets the required specifications.
### Step 3: Rinsing and Drying
Thorough rinsing of stainless steel components post-passivation is vital to eliminate lingering chemicals, crucial for surface purity. Subsequent drying is pivotal to prevent water spot formation and corrosion, preserving metal integrity during the process. Adhering to meticulous rinsing and drying protocols significantly enhances the efficacy of the passivation process by removing all remnants of chemicals, safeguarding metal durability. Complete removal of chemical substances through rinsing is imperative for sustained protection. Thorough drying maintains the integrity of the crucial oxide layer that is formed during passivation.

## Challenges of Passivation
Despite its benefits, challenges may arise, such as the need for regular intervals of sodium dichromate treatments to uphold the passivation layer’s integrity. Manufacturers must be aware of potential issues that can occur during passivation, necessitating a thorough understanding of conversion coatings applied in such cases.
### Potential Issues and Solutions
During passivation, issues like corrosion or discoloration can occur, especially if not performed correctly. Selecting the appropriate passivation method based on the stainless steel type and part's purpose is critical. To avoid problems, thorough cleaning and proper handling before and after passivation are recommended. Partnering with experienced passivation providers ensures the effectiveness of the process, leading to optimal results.
## Safety Measures During Passivation
Safety precautions are crucial during the passivation of stainless steel due to the use of different chemicals that can be potentially hazardous. Ensure proper personal protective equipment, like gloves and eye protection. Work in well-ventilated spaces to prevent inhalation of fumes. Strictly adhere to manufacturer guidelines for solution concentrations. Thoroughly rinse components post-passivation to eliminate any leftover chemicals. Dispose of solutions and rinse water according to local regulations to maintain safety standards.
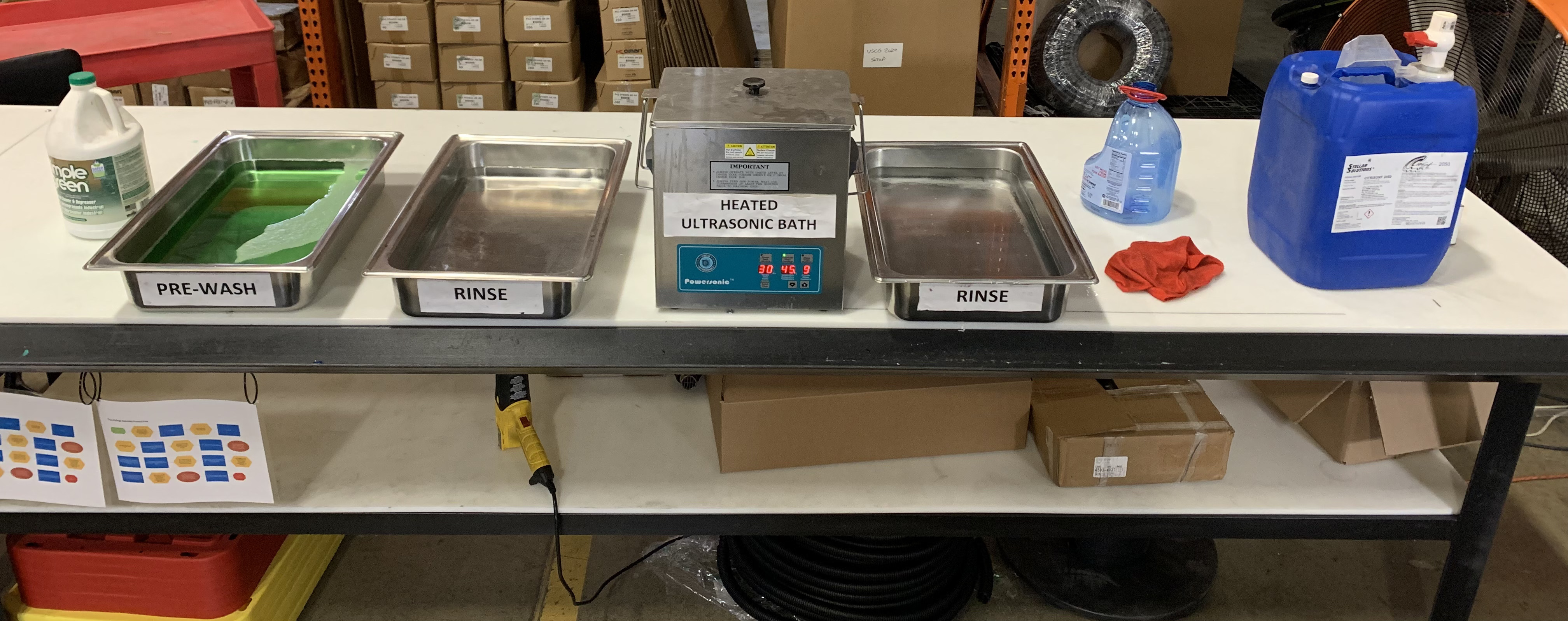
## Essential Equipment for Stainless Steel Passivation
In the realm of stainless steel passivation, a range of specialized equipment is essential for ensuring both the efficiency and effectiveness of the process. Central to this are acid-resistant passivation tanks or baths equipped with temperature control systems for immersing parts in the solution. Following passivation, spray rinse stations and drying ovens ensure the complete removal of residual acids and moisture. Automated handling systems, including conveyors and robotic arms, facilitate smooth transitions between passivation, rinsing, and drying stages. Pre-passivation cleaning may involve ultrasonic cleaners for thorough decontamination. The process's integrity is monitored using tools like pH meters and solution analyzers, while the safety of operators is ensured through appropriate personal protective equipment (PPE).
## Frequently Asked Questions
### What is passivation?
Passivation involves a chemical process that removes free iron from stainless steel surfaces, enhancing their corrosion resistance with a protective oxide layer. This technique prevents rust, staining, and corrosion, crucial for industries needing durable components.
### Does stainless steel need passivation?
Stainless steel benefits from passivation. This process helps ensure the longevity and corrosion resistance of stainless steel components. It's recommended to passivate stainless steel after fabrication or machining to enhance its performance and durability.
### What chemicals are used in passivation?
The chemicals commonly used in passivation include citric acid, nitric acid, and sodium dichromate. These chemicals help clean the metal surface, remove impurities, and promote the formation of the protective oxide layer.
### Can I passivate carbon steel?
The passivation of carbon steel involves applying external protective coatings or treatments to prevent corrosion, unlike the natural oxide layer formation in stainless steel. Techniques include oiling, painting, or other corrosion inhibitors, and sometimes controlled oxidation processes. These coatings act as barriers against environmental elements but are more prone to damage and wear, requiring regular maintenance and reapplication, unlike the self-healing chromium oxide layer on stainless steel.
### What Factors Influence the Success of the Passivation Process?
1) Chromium content vital for the oxide layer
2) Correct immersion duration
3) Consideration of surface grain boundaries
4) Maintaining ideal conditions like temperature and solution strength
5) Recognizing unique requirements for varied metals
Shop our Stainless Steel Compression Fittings
This article provides an overview of the passivation process, its importance, and practical applications, aiming to help readers understand the science and significance of passivation in enhancing the durability and performance of stainless steel components.