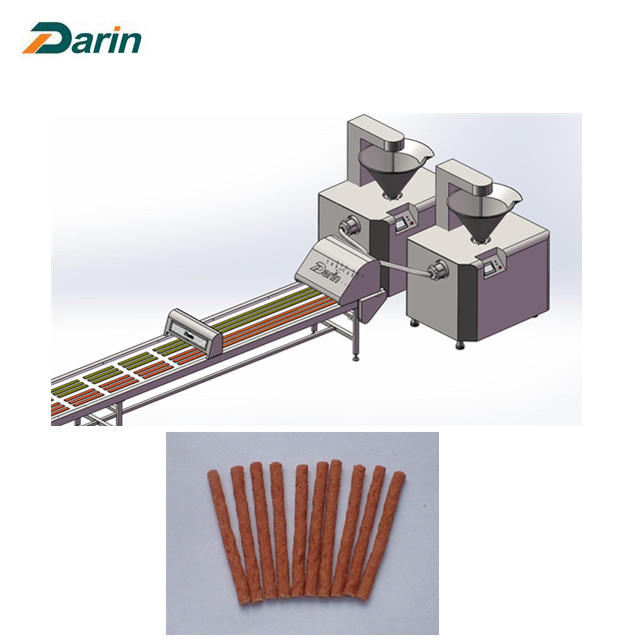
For medical devices, what people are most concerned about is the function and safety information. The way that most people obtain this information is nothing more than the manufacturers, doctors, and medical management departments. Auto Meat Strips Processing Line is latest design of Darin, which could produce pet snacks, suitable for pet food without casing directly baked meat and meat products, widely used in pet food article beef, article chicken, beef rod, chicken rod production and processing.
7, Safely cutting system to ensure the worker's safety, also same size of meat strips.
Auto Meat Strip Processing Line Jerky Treats Stick Machine,Auto Meat Strip Processing Line,Meat Stick Making Machine,Dog Chewing Stick Machine Jinan Darin Machinery Co., Ltd. , https://www.globaldarin.com
Recently, in order to better disclose such information to the public, FDA has upgraded its openFDA application process interface. This also means that in the future, app developers can develop a mobile app to specifically look for device classification, registration time, recall situation, and adverse events.
FDA staff said it was also the largest upgrade of openFDA since its launch last year. The FDA has deposited 6,000 equipment classification records, 24,000 company location registration information and 100,000 company product lists on the system.
In addition, the information included more than 30,000 listing approval resolutions for equipment, the earliest of which dates back to 1976. At the same time, app developers can also obtain 9,500 recall records since 2002 and 4.2 million adverse reaction reports from 1991 to the present.
The FDA said that although some of the data have already been opened to the public, the FDA hopes to use the openFDA update to present it to consumers in a more convenient form for their use. The popularity of smart phones has undoubtedly provided an excellent opportunity for the FDA's efforts.
The pharmaceutical management department is constantly improving the examination and approval system for drugs and medical devices, and it is equally important to disclose and disclose information in a timely manner. On the one hand, consumers can access relevant data in a timely manner, understand the latest product trends, and can be promptly pushed to relevant consumers in the event of adverse reactions; on the other hand, pharmaceutical industry practitioners can also integrate these data to make further developments for the future. Plan well. In this regard, China's pharmaceutical administration may still have a lot to learn.
1, It is first processing line to produce meat snacks automatically and continuously.
2, The diameter and shape of meat strips can be various by changing the molds.
3, Fully controlled by PLC and touch screen to reduce the labor cost, 2-3 workers are needed to operate the whole line.
4, The characteristics is discharging speed, output evenly, can infuse bowel flesh lump, can also be used to fill the mi bowel, flexible, convenient and free loading, high efficiency, continuous production capacity, and cleaning convenient, suitable for various types of production enterprises.
5, The equipment is made from high quality 304 stainless steel, corrosion resistance, with high quality in intensity, reliable and durable, convenient cleaning, adopt gear two-way extrusion type structure, with high pressure, rotation smoothly.
6, Equipped with Auto Tray Loading System to feed the tray to load the meat strip automatically.